Detection of TTR Amyloid in the Conjunctiva Using a Novel Fluorescent Ocular Tracer

February 18, 2024 – Background: Transthyretin amyloidosis (ATTR) is a significant cause of cardiomyopathy and other morbidities in the elderly and Black Americans. ATTR can be treated with new disease-modifying therapies, but large shortfalls exist in its diagnosis. The objective of this study was to test whether TTR amyloid can be detected and imaged in the conjunctiva using a novel small-molecule fluorescent ocular tracer, with the implication that ATTR might be diagnosable by a simple eye examination.
Methods: Three approaches were used in this study. First, AMDX-9101 was incubated with in vitro aggregated TTR protein, and changes in its excitation and emission spectra were quantified. Second, a cadaver eye from a patient with familial amyloid polyneuropathy type II TTR mutation and a vitrectomy sample from an hATTR patient were incubated with AMDX-9101 and counterstained with Congo Red and antibodies to TTR to determine whether AMDX-9101 labels disease-related TTR amyloid deposits in human conjunctiva and eye. Last, imaging of in vitro aggregated TTR amyloid labeled with AMDX-9101 was tested in a porcine ex vivo model, using a widely available clinical ophthalmic imaging device.
Results: AMDX-9101 hyper-fluoresced in the presence of TTR amyloid in vitro, labeled TTR amyloid deposits in postmortem human conjunctiva and other ocular tissues and could be detected under the conjunctiva of a porcine eye using commercially available ophthalmic imaging equipment.
Conclusions: AMDX-9101 enabled detection of TTR amyloid in the conjunctiva, and the fluorescent binding signal can be visualized using commercially available ophthalmic imaging equipment.
Translational relevance: AMDX-9101 detection of TTR amyloid may provide a potential new and noninvasive test for ATTR that could lead to earlier ATTR diagnosis, as well as facilitate development of new therapeutics.
Conflict of interest statement
Disclosure: J. Pilotte, Amydis (E); A.S. Huang, Amydis (F); S. Khoury, Amydis (E); X. Zhang, None; A. Tafreshi, Amydis (C); P. Vanderklish, Amydis (E); S.T. Sarraf, Amydis (O); J.S. Pulido, Amydis (F); T. Milman, None
Figures:
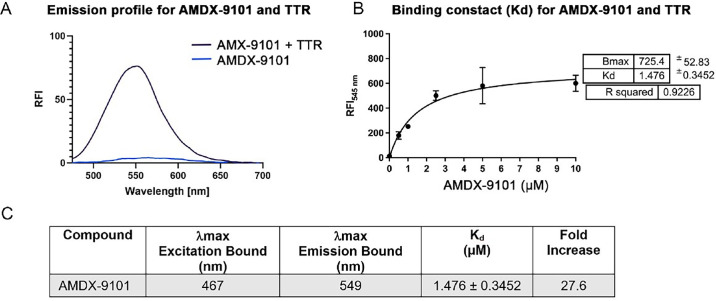
Figure 1. Spectroscopic and binding properties of AMDX-9101 combined with aggregated wild type TTR. (A) Fluorescent emission of AMDX-9101 before (blue) and after (purple) mixing with TTR aggregates. (B) Plot of the fluorescence intensity (λmax = 545 nm) as a function of the concentration of AMDX-9101 in the presence of aggregated TTR peptides (5 µM) in solution. Fitting this data to the equation: y = Bx/(Kd + x) revealed a Kd of 1.476 ± 0.3452 µM for association of AMDX-9101 to aggregated TTR peptides. (C) Spectroscopic properties of AMDX-9101 and wt TTR. Fold increase determined from the emission with 4 µM AMDX and 5 µM aggregated TTR at the maximum emission of AMDX + TTR.
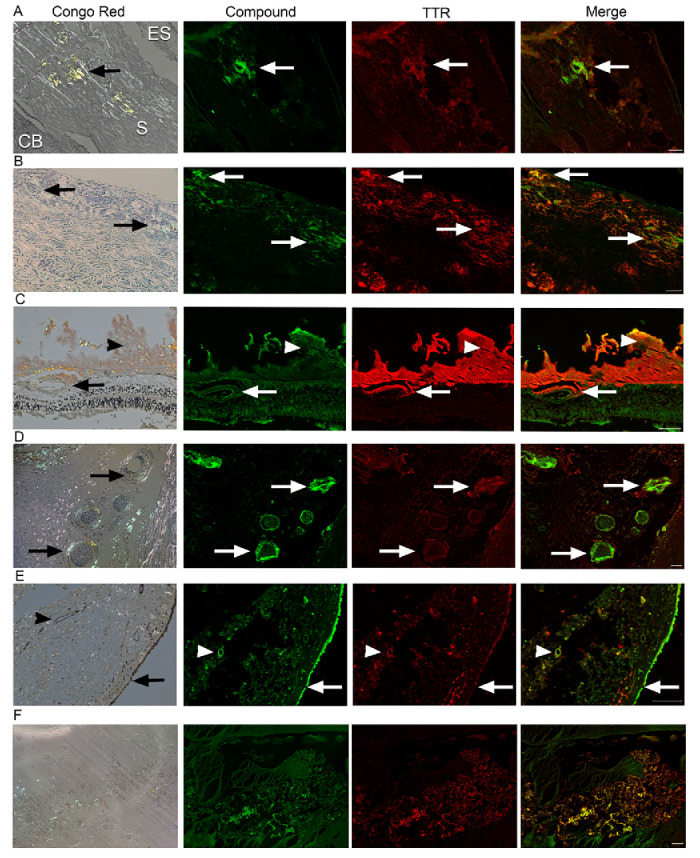
Figure 2. Ex vivo co-staining of AMDX-9101 and TTR in human ATTR amyloidosis eye. FFPE ocular sections from a human ATTR amyloidosis eye were either (1) stained with Congo red and examined in bright and polarized light using a Leica microscope equipped with a polarizer and analyzer. The characteristic green birefringent polarization color was taken as proof of the presence of amyloid in the tissue (left column) or (2) FFPE sections were co-stained with AMDX-9101 (green) and TTR antibody (red). Overlay of all channels shows colocalization in yellow. Scale bar = 100 µm. Images taken from 87-year-old man with FAP type II TTR mutation (serine 84) TTR amyloidosis: (A) Sclera adjacent to emissarial canals (ES, episclera; S, sclera; CB, ciliary body; arrows, amyloid); (B) Optic nerve sheath (arrows, amyloid); (C) Retina and vitreous, amyloid deposits around retinal vessels (arrows) and in the vitreous (arrowhead); (D) Posterior ciliary arteries and nerves (arrows); (E) Conjunctiva, epithelium (arrows), and vessels in the stroma (arrowheads). (F) Vitreous amyloid from an hATTR patient (TTR c.148G>A; p.Val50Met heterozygous missense, autosomal dominant).
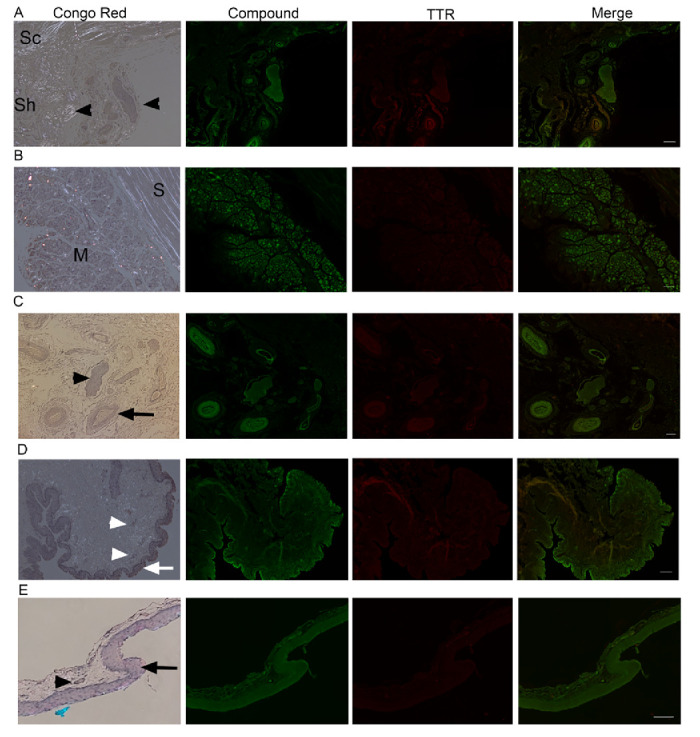
Figure 3. Ex vivo ocular images in human controls. FFPE ocular sections from human control eyes were either stained with Congo red and examined in bright and polarized light using a Leica microscope equipped with a polarizer and analyzer (the characteristic green birefringent polarization color was taken as a proof of the presence of amyloid in the tissue, left column) or co-stained with AMDX-9101 (green) and the TTR antibody (red). Overlay of all channels shows absence of colocalization in yellow. Scale bar = 100 µm. (A–C) From a 62-year-old patient with uveal melanoma: (A) optic nerve sheath (Sh), sclera (Sc), and posterior ciliary arteries/nerves (arrowheads). Note intrinsic yellow-white birefringence of the collagen, (B) sclera (S), and extraocular muscles (M), and (C) posterior ciliary arteries (arrow) and nerves (arrowhead). (D) Conjunctiva from three-year-old patient, epithelium (arrow), vessels in the stroma (arrowheads). (E) Conjunctiva from healthy adult, epithelium (arrow), vessel (arrowheads).
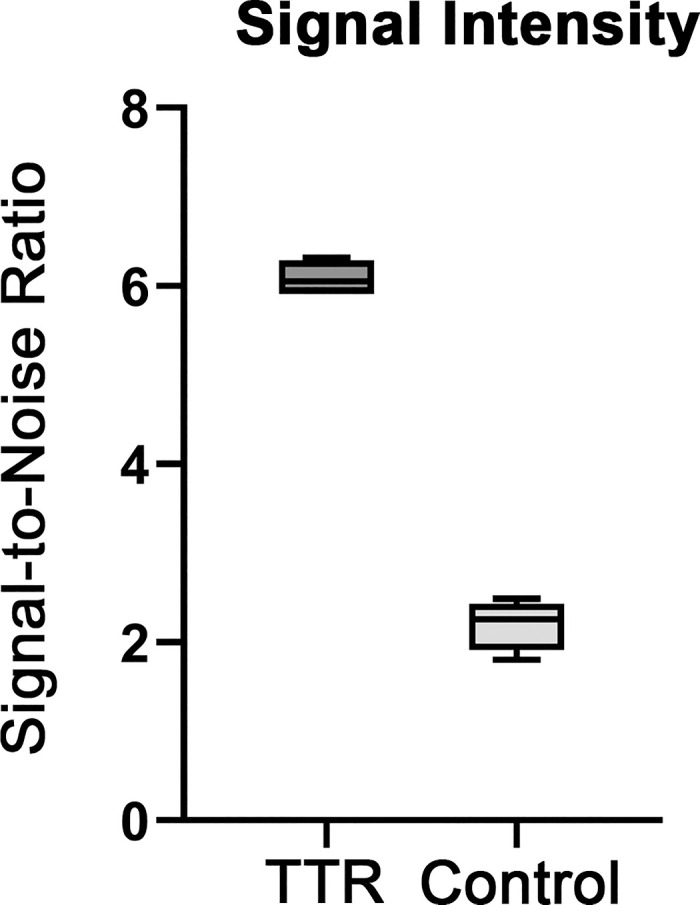
Figure 4. Quantification of AMDX-9101 signal intensity. Regions were selected from four separate fields. All images were analyzed by MATLAB R2022b. The fluorescent signal-to-noise ratio was calculated as the average of the foreground pixels over the average of the background pixels. Results were plotted as box plots, indicating the twenty-fifth percentile (bottom boundary), median (middle line), seventy-fifth percentile (top boundary), and nearest observations within 1.5 times the interquartile range (whiskers).
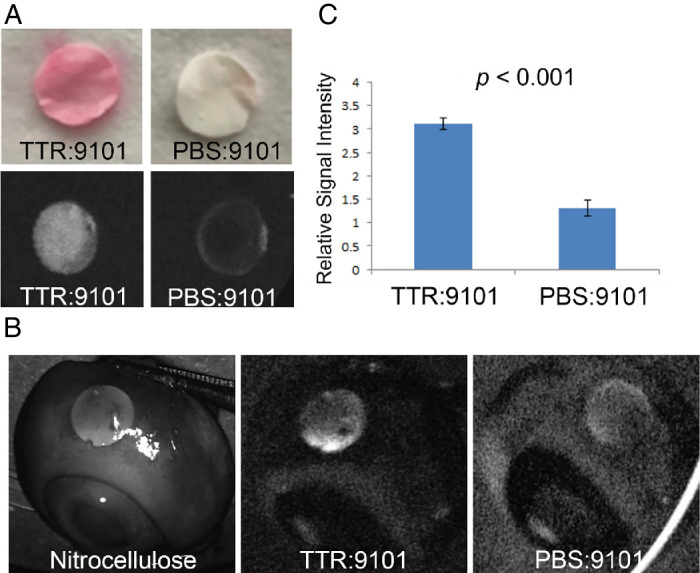
Figure 5. Visualization of TTR with AMDX-9101 in an ex vivo porcine eye model. (A) Nitrocellulose membrane discs (NMDs) were cut and incubated with TTR protein and AMDX-9101 and stained with Ponceau-S (top panel). Fluorescence images were taken using a Spectralis angiographic camera, demonstrating fluorescence with AMDX-9101. (B)
Julie Pilotte 1, Alex S Huang 2, Sami Khoury 1, Xiaowei Zhang 2, Ali Tafreshi 1, Peter Vanderklish 1, Stella T Sarraf 1, Jose S Pulido 3, Tatyana Milman 3, 4
1 Amydis, Inc., La Jolla, CA, USA.
2 Hamilton Glaucoma Center, Shiley Eye Institute, Viterbi Family Department of Ophthalmology, University of California San Diego, La Jolla, CA, USA.
3 Vickie and Jack Farber Vision Research Center and MidAtlantic Retina Service, Wills Eye Hospital, Philadelphia, PA, USA.
4 Pathology Department, Wills Eye Hospital, Philadelphia, PA, USA.
References
- Andrade C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain . 1952; 75: 408–427. – PubMed
- Muchtar E, Dispenzieri A, Magen H, et al. .. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med . 2021; 289: 268–292. – PubMed
- Koike H, Katsuno M. Transthyretin amyloidosis: update on the clinical spectrum, pathogenesis, and disease-modifying therapies. Neurol Ther . 2020; 9: 317–333. – PMC – PubMed
- Cohen OC, Wechalekar AD. Systemic amyloidosis: moving into the spotlight. Leukemia . 2020; 34: 1215–1228. – PubMed
- Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet . 2016; 387(10038): 2641–2654. – PubMed
- Plante-Bordeneuve V. Transthyretin familial amyloid polyneuropathy: an update. J Neurol . 2018; 265: 976–983. – PubMed
- Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS.. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol . 2019; 73: 2872–2891. – PMC – PubMed
- Adams D, Koike H, Slama M, Coelho T.. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol . 2019; 15: 387–404. – PubMed
- Benson MD, Buxbaum JN, Eisenberg DS, et al. .. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid . 2018; 25: 215–219. – PubMed
- Sanguinetti C, Minniti M, Susini V, et al. .. The journey of human transthyretin: synthesis, structure stability, and catabolism. Biomedicines . 2022; 10: 1906. – PMC – PubMed
- Jacobsson B. Localization of transthyretin-mRNA and of immunoreactive transthyretin in the human fetus. Virchows Arch A Pathol Anat Histopathol . 1989; 415: 259–263. – PubMed
- Cavallaro T, Martone RL, Dwork AJ, Schon EA, Herbert J. The retinal pigment epithelium is the unique site of transthyretin synthesis in the rat eye. Invest Ophthalmol Vis Sci . 1990; 31: 497–501. – PubMed
- Su Y, Jono H, Misumi Y, et al. .. Novel function of transthyretin in pancreatic alpha cells. FEBS Lett . 2012; 586: 4215–4222. – PubMed
- Gertz MA. Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges. Am J Manag Care . 2017; 23(7 Suppl): S107–S112. – PubMed
- Adam MP, Mirzaa GM, Pagon RA, et al.., eds. GeneReviews. Seattle: University of Washington, Seattle, 1993.
- Pitkänen P, Westermark P, Cornwell GG 3rd. Senile systemic amyloidosis. Am J Pathol . 1984; 117: 391–399. – PMC – PubMed
- Obi CA, Mostertz WC, Griffin JM, Judge DP. ATTR Epidemiology, genetics, and prognostic factors. Methodist Debakey Cardiovasc J . 2022; 18(2): 17–26. – PMC – PubMed
- Koike H, Misu K-I, Ikeda S-I, et al. .. Type I (Transthyretin Met30) familial amyloid polyneuropathy in Japan: early- vs late-onset form. Arch Neurol . 2002; 59: 1771–1776. – PubMed
- Misu K, Hattori N, Nagamatsu M, et al. .. Late-onset familial amyloid polyneuropathy type I (transthyretin Met30-associated familial amyloid polyneuropathy) unrelated to endemic focus in Japan. Clinicopathological and genetic features. Brain . 1999; 122(Pt 10): 1951–162. – PubMed
- Palladini G. Wild type ATTR amyloidosis. Available at: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=330001. Accessed January 08, 2023.
- Porcari A, Fontana M, Gillmore JD. Transthyretin cardiac amyloidosis. Cardiovasc Res . 2023; 118: 3517–3535. – PMC – PubMed
- Antonopoulos AS, Panagiotopoulos I, Kouroutzoglou A, et al. .. Prevalence and clinical outcomes of transthyretin amyloidosis: a systematic review and meta-analysis. Eur J Heart Fail . 2022; 24: 1677–1696. – PubMed
- Koike H, Katsuno M.. Ultrastructure in transthyretin amyloidosis: from pathophysiology to therapeutic insights. Biomedicines . 2019; 7(1): 11. – PMC – PubMed
- González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. .. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J . 2015; 36: 2585–2594. – PubMed
- Buxbaum JN, Ruberg FL. Transthyretin V122I (pV142I)* cardiac amyloidosis: an age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med . 2017; 19: 733–742. – PMC – PubMed
- Maurer MS, Hanna M, Grogan M, et al. .. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol . 2016; 68: 161–172. – PMC – PubMed
- Irabor B, McMillan JM, Fine NM.. Assessment and management of older patients with transthyretin amyloidosis cardiomyopathy: geriatric cardiology, frailty assessment and beyond. Front Cardiovasc Med . 2022; 9: 863179. – PMC – PubMed
- Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN.. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid . 2015; 22: 171–174. – PubMed
- Alexander KM, Falk RH.. V122I TTR cardiac amyloidosis in patients of African descent: recognizing a missed disease or the dog that didn’t bark? Circ Heart Fail . 2016; 9(9): e003489. – PubMed
- Chandrashekar P, Alhuneafat L, Mannello M, et al. .. Prevalence and outcomes of p.Val142Ile TTR amyloidosis cardiomyopathy: a systematic review. Circ Genom Precis Med . 2021; 14(5): e003356. – PMC – PubMed
- Qin Q, Wei C, Piao Y, et al. .. Current review of leptomeningeal amyloidosis associated with transthyretin mutations. Neurologist . 2021; 26: 189–195. – PMC – PubMed
- Beirão JM, Malheiro J, Lemos C, Beirão I, Costa P, Torres P.. Ophthalmological manifestations in hereditary transthyretin (ATTR V30M) carriers: a review of 513 cases. Amyloid . 2015; 22: 117–122. – PMC – PubMed
- Dammacco R, Merlini G, Lisch W, et al. .. Amyloidosis and ocular involvement: an overview. Semin Ophthalmol . 2020; 35: 7–26. – PubMed
- Haraoka K, Ando Y, Ando E, et al. .. Amyloid deposition in ocular tissues of patients with familial amyloidotic polyneuropathy (FAP). Amyloid . 2002; 9: 183–189. – PubMed
- Ioannou A, Fontana M, Gillmore JD. RNA targeting and gene editing strategies for transthyretin amyloidosis. BioDrugs . 2023; 37: 127–142. – PMC – PubMed
- Tsoi MR, Lin JH, Patel AR. Emerging therapies for transthyretin amyloidosis. Curr Oncol Rep . 2023; 25: 549–558. – PubMed
- Gonzalez-Lopez E, Escobar-Lopez L, Obici L, et al. .. Prognosis of transthyretin cardiac amyloidosis without heart failure symptoms. JACC CardioOncol . 2022; 4: 442–454. – PMC – PubMed
- Cruz Rodriguez JB, Tallaj JA. Narrative review of pharmacotherapy for transthyretin cardiac amyloid. Ann Transl Med . 2021; 9: 519. – PMC – PubMed
- Park J, Egolum U, Parker S, Andrews E, Ombengi D, Ling H. Tafamidis: a first-in-class transthyretin stabilizer for transthyretin amyloid cardiomyopathy. Ann Pharmacother . 2020; 54: 470–477. – PubMed
- Rozenbaum MH, Large S, Bhambri R, et al. .. Impact of Delayed diagnosis and misdiagnosis for patients with transthyretin amyloid cardiomyopathy (ATTR-CM): a targeted literature review. Cardiol Ther . 2021; 10: 141–159. – PMC – PubMed
- Vera-Llonch M, Reddy SR, Chang E, Tarbox MH, Pollock M.. The patient journey toward a diagnosis of hereditary transthyretin (ATTRv) amyloidosis. Orphanet J Rare Dis . 2021; 16(1): 25. – PMC – PubMed
- Gertz M, Adams D, Ando Y, et al. .. Avoiding misdiagnosis: expert consensus recommendations for the suspicion and diagnosis of transthyretin amyloidosis for the general practitioner. BMC Fam Pract . 2020; 21: 198. – PMC – PubMed
- Maurer MS, Bokhari S, Damy T, et al. .. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail . 2019; 12(9): e006075. – PMC – PubMed
- Vergaro G, Solal AC, Emdin M.. Wild type transthyretin amyloidosis: don’t miss diagnosis! Int J Cardiol . 2020; 312: 96–97. – PubMed
- Galant NJ, Westermark P, Higaki JN, Chakrabartty A.. Transthyretin amyloidosis: an under-recognized neuropathy and cardiomyopathy. Clin Sci . 2017; 131: 395–409. – PubMed
- Frangolho A, Correia BE, Vaz DC, Almeida ZL, Brito RMM.. Oligomerization profile of human transthyretin variants with distinct amyloidogenicity. Molecules . 2020; 25(23): 5698. – PMC – PubMed
- Akiyama G, Saraswathy S, Bogarin T, et al. .. Functional, structural, and molecular identification of lymphatic outflow from subconjunctival blebs. Exp Eye Res . 2020; 196: 108049. – PMC – PubMed
- Lee JY, Heilweil G, Le P, et al. .. Structural confirmation of lymphatic outflow from subconjunctival blebs of live human subjects. Ophthalmol Sci . 2021; 1(4): 100080. – PMC – PubMed
- Aguilar-Calvo P, Sevillano AM, Rasool S, et al. .. Noninvasive antemortem detection of retinal prions by a fluorescent tracer. J Alzheimers Dis . 2022; 88: 1137–1145. – PMC – PubMed
- Ferreira E, Almeida ZL, Cruz PF, Silva ESM, Veríssimo P, Brito RMM.. Searching for the best transthyretin aggregation protocol to study amyloid fibril disruption. Int J Mol Sci . 2021; 23(1): 391. – PMC – PubMed
- Damrauer SM, Chaudhary K, Cho JH, et al. .. Association of the v122i hereditary transthyretin amyloidosis genetic variant with heart failure among individuals of African or Hispanic/Latino ancestry. JAMA . 2019; 322: 2191–2202. – PMC – PubMed
- Spencer-Bonilla G, Njoroge JN, Pearson K, Witteles RM, Aras MA, Alexander KM. Racial and ethnic disparities in transthyretin cardiac amyloidosis. Curr Cardiovasc Risk Rep . 2021; 15(6): 8. – PMC – PubMed
- Davies DR, Redfield MM, Scott CG, et al. .. A simple score to identify increased risk of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. JAMA Cardiol . 2022; 7: 1036–1044. – PMC – PubMed
- Longhi S, Guidalotti PL, Quarta CC, et al. .. Identification of TTR-related subclinical amyloidosis with 99mTc-DPD scintigraphy. JACC Cardiovasc Imaging . 2014; 7: 531–532. – PubMed
- Mohamed-Salem L, Santos-Mateo JJ, Sanchez-Serna J, et al. .. Prevalence of wild type ATTR assessed as myocardial uptake in bone scan in the elderly population. Int J Cardiol . 2018; 270: 192–196. – PubMed
- Castano A, Haq M, Narotsky DL, et al. .. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol . 2016; 1: 880–889. – PubMed
- Bokhari S, Castaño A, Pozniakoff T, Deslisle S, Latif F, Maurer MS.. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging . 2013; 6: 195–201. – PMC – PubMed
- Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D.. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail . 2018; 24: 131–133. – PubMed
- Milandri A, Farioli A, Gagliardi C, et al. .. Carpal tunnel syndrome in cardiac amyloidosis: implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur J Heart Fail . 2020; 22: 507–515. – PubMed
- Boyle RP, Sharan J, Schwartz G.. Carpal tunnel syndrome in transthyretin cardiac amyloidosis: implications and protocol for diagnosis and treatment. Cureus . 2021; 13(4): e14546. – PMC – PubMed
- Fosbøl EL, Rørth R, Leicht BP, et al. .. Association of carpal tunnel syndrome with amyloidosis, heart failure, and adverse cardiovascular outcomes. J Am Coll Cardiol . 2019; 74: 15–23. – PubMed
- Quarta CC, Gonzalez-Lopez E, Gilbertson JA, et al. .. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J . 2017; 38: 1905–1908. – PMC – PubMed
- Cohen OC, Sharpley F, Gilbertson JA, et al. .. The value of screening biopsies in light-chain (AL) and transthyretin (ATTR) amyloidosis. Eur J Haematol . 2020; 105: 352–356. – PubMed
- Ehrlich JR, Ndukwe T, Solway E, et al. .. Self-reported eye care use among US adults aged 50 to 80 years. JAMA Ophthalmol . 2019; 137: 1061–1066. – PMC – PubMed
- Bazargan M, Ekwegh T, Cobb S, Adinkrah E, Assari S. Eye Examination recency among African American older adults with chronic medical conditions. Healthcare (Basel) . 2020; 8(2): 94. – PMC – PubMed
- Lee CS, Morris A, Van Gelder RN, Lee AY. Evaluating access to eye care in the contiguous United States by calculated driving time in the United States Medicare population. Ophthalmology . 2016; 123: 2456–2461. – PMC – PubMed